A Large-Scale Breakup
- Punam Medh

- May 7, 2025
- 14 min read
Updated: Nov 30, 2025

In a flash the world changed forever!
On August 6, 1945, the United States dropped the first-ever atomic bomb on the Japanese city of Hiroshima. The bomb, known as ‘Little Boy, was detonated at 8:15 a.m., unleashing unparalleled destruction. Three days later, on August 9, 1945, a second bomb, ‘Fat Man’, was dropped on Nagasaki.
These bombings caused immense casualties and devastation. A week later, on August 15, 1945, Japan announced its surrender, effectively bringing an end to World War II.
The development of the atomic bomb was the culmination of the Manhattan Project, a massive scientific and military effort involving some of the greatest minds of the time, such as Robert Oppenheimer, Enrico Fermi, Hans Bethe and Leo Szilard, to name just a few. This ground breaking development stemmed from the discovery that the nuclei of heavy atoms, like uranium or plutonium, once thought to be indivisible, could actually be split when bombarded with slow-moving neutrons.
This process of splitting the nucleus is known as nuclear fission, where a heavy atomic nucleus breaks into two smaller fragments of roughly equal mass, releasing a tremendous amount of energy. The events leading to this discovery makes for one of the most captivating stories of scientific breakthrough in the history of science.
Among the pioneers whose work laid the foundation for this breakthrough discovery was Lise Meitner, a theoretical physicist who also mastered experimental techniques. This is a story about her, her exceptional skills as a scientists and her unparalleled character as a human being.
Lise Meitner’s Early Life
Lise Meitner was born in 1878 in Vienna, Austria, into a cultured and progressive Jewish family that valued education. Her father, one of Austria’s first Jewish lawyers, strongly believed in intellectual development and ensured that she had access to learning. Education for girls in Austria however was limited. After completing public school at the age of fourteen, she had no access to further education.
When the university finally admitted women, Meitner prepared for the Matura (university entrance exam) with the help of private tutors, compressing eight years of missed schooling into just two. Rontgen’s 1895 discovery Becquerel’s finding of uranium radiation followed in 1896 had fascinated an eighteen-year old Meitner. She was determined that the study of radioactivity would be her path in life whether or not the prevailing norms of society permitted it.
At 23, she enrolled at the University of Vienna, five years older than most of her classmates. Facing a male-dominated scientific world, she developed a relentless work ethic, dedicating long hours to lectures and lab work, determined to succeed in physics.
Amir Aczel (2009), in the book ‘Uranium Wars’ shares an interesting anecdote about Meitner. One day, Meitner’s calculus professor, recognizing her exceptional talent, decided to challenge her. He handed her an article by an Italian mathematician and asked her to find the error hidden within it. Meitner took on the task eagerly, quickly spotting the mistake. Impressed, the professor encouraged her to publish her findings. But Meitner hesitated. To her, it didn’t feel right because the professor had already known the error was there; he had simply led her to it. Publishing the correction as her own felt dishonest. She politely declined, insisting that credit belonged to him instead.
It was a small moment, but one that revealed that no matter how great the opportunity, she never sought recognition for work she did not truly see as her own.
Meitner’s doctoral research was on heat conduction. It involved complex experimental setups like – using mercury droplets suspended in fat molecules. She chose an experimental project rather than a theoretical one because her earlier studies had been largely confined to theory, and she wanted hands-on laboratory experience to strengthen her scientific foundation. Her doctoral work honed her expertise in designing sophisticated experimental setups, an ability that would define her later work in radioactivity.
In 1906, Meitner became the second woman to earn a doctorate in physics from the University of Vienna. After her graduation, she found herself facing a situation, not uncommon amongst women scientists of those times – finding a suitable position in a lab. In order not to have to leave physics altogether, Meitner took a job for which she was overqualified, that of instructor at a girls school. But at night she continued her research work in the university’s physics laboratories.
Meitner’s early life, provides glimpses of ambition and determination combined with unbounded curiosity for understanding the nature of the physical word. She had a sharp scientific mind, excelling in both theoretical and experimental physics, but what set her apart was her quiet integrity. Meitner carved a path for herself in a world that was not ready to make space for women in science.
Radioactivity Rising
Radioactivity was a new and emerging field at the turn of the 20th century. In 1906, ten years after the discovery of radioactivity, the number of known radioactive species stood at over 20 and was rising fast. Very little as known about radioactivity and scientists were focusing on identifying new radioactive elements. The field was chaotic, yet inviting, because there was so much to discover. What exactly was radioactivity? What made an element radioactive? What was the nature of radioactivity? All any scientist needed as a newcomer was a radioactivity measuring device like a photographic plate or an electroscope and a radioactive source.
Meitner’s part-time research involved studying how alpha particles passed through different metals using an electroscope. She confirmed that heavier metals scattered more particles, a finding that supported the work of Curie and Rutherford. Her results were published in a journal
in 1907.
Armed with this brief experience, she moved from Vienna to Berlin in 1907 in search of research opportunities. Berlin at that time was the hub of innovation, attracting researchers from all over the world. Pioneering scientists like Max Planck and Albert Einstein conducted their research here because the city offered a vibrant academic environment, substantial funding from institutions like the Kaiser Wilhelm Society and an intellectual ecosystem that fostered ground breaking discoveries.
Meitner’s Foray into Research
The turn of 20th century marked a long and rich harvest of ideas in physics and chemistry. Two fundamental discoveries – the atomic nucleus in 1911 by Rutherford and isotopes in 1913 – added to the zeal and spirit of scientific achievement. Scientists realized that there was a discovery from the labs working in these areas across Paris, Berlin, London and Prague. Between 1913-14, new models of the atom emerged and challenged the existing knowledge of the periodic table.
It is in these exciting times that Lise Meitner entered the field of radioactive studies. In Berlin, she met Otto Hahn, a skilled scientist and chemist. heir shared fascination with radioactivity sparked an immediate connection. Hahn, a chemist, lacked a background in physics and mathematics needed to fully grasp adioactivity, while Meitner, a physicist, had a limited foundation in chemistry. Their skills complemented each other perfectly, creating a partnership that bridged the gap between the two subjects. Together, they embarked on an ambitious research project, one that aimed to uncover the secrets of radiation and reveal how elements transformed under its influence.
At the University of Berlin, where Hahn was working, Meitner was also offered a research position. The atmosphere around her however was hostile, she was excluded from the main laboratories of the building and had to use a small wood workshop for her experiments. And still, her collaboration with Hahn was productive, leading to several publications.
Another anecdote that serves as an example of how women were looked upon in science took place, this time with Ernest Rutherford. He was on his way to England after receiving his Nobel Prize from Stockholm and decided to stop at Berlin and visit their university. When he was introduced to Meitner, whose name he had only seen on publications as ‘Meitner, L.’ exclaimed “Oh, I thought you were a man!”. A telling tale of the status of women in science.
Despite this discrimination, Meitner had an had an ever-positive outlook on life, and a gift for forming new friendships and keeping friends for life.
The Discovery of Protactinium
The discovery of a new element, protactinium (91) sets the stage for the remarkable mutually beneficial collaboration between Meitner and Hahn.
Meitner and Hahn were investigating the origins of actinium, a mysterious element always found near uranium, suggesting that it was part of uranium’s decay chain. But there had to be an unknown element, a ‘parent’ that decayed into actinium. Based on radioactive decay laws and the periodic table, they predicted that this missing element had an atomic number of 91, sitting between thorium (90) and uranium (92).
To track it down, they designed a careful experiment using two uranium sources: an old uranium nitrate sample that had been sitting in the lab for years and fresh pitchblende ore. Their reasoning was simple but required patience. Since many radioactive elements decay quickly, they hoped that by waiting, the short-lived isotopes would vanish, leaving behind the more stable ones including the elusive element 91. Over time, the amount of actinium would rise, revealing patterns in radiation that could point to its parent.
Their persistence paid off. After careful observation and chemical isolation, they uncovered the missing element, protactinium (Pa), the true parent of actinium. The discovery not only filled a gap in the periodic table but also proved the power of radioactive decay laws and meticulous experimentation.
This journey spread over seven years, was interrupted by World War I. Hahn had to serve in the war and Meitner volunteered Meitner volunteered to help tend the wounded in field hospitals as an X-ray technician, allowing them to work together only when they could both get leave.
The Transuranic Race
In 1932 James Chadwick discovered the neutron. It was a crucial piece in the puzzle that allowed Heisenberg (along with other physicists) to develop a more complete and accurate model of the atomic nucleus, which included protons and neutrons. to propose a new model of the nucleus, suggesting that it is made up of neutrons and protons.
And then came the one experiment by Enrico Fermi, an Italian physicist who excelled in theoretical and experimental physics. He recognized that neutrons were an ideal projectile for nuclear reactions. Their lack of electrical charge would allow them to penetrate atomic nuclei more easily compared to charged particles like the positively charged protons or alpha particles which would be repelled by the positively charged nucleus. He and his colleagues focused on bombarding the nuclei of Uranium and other heavy elements in the periodic table.
The periodic table at this point extended up to the heaviest element known then: uranium (92). Initially, he hypothesized that the absorption of neutrons by elements like uranium resulted in the formation of heavier elements which he called transuranic. He believed that the uranium nucleus was absorbing the neutrons and then undergoing beta decay to form these heavier elements. The possibility of creating transuranic elements through neutron absorption, sparked considerable interest. Although Fermi later revised his interpretation after the discovery of nuclear fission, his pioneering work laid the foundation for what became known as the race to discover elements heavier than uranium – aptly called the transuranic race.
Initially, Fermi’s findings were ‘erroneously confirmed’ by a number of scientists including Meitner and Hahn. She had been following Fermi’s experiments closely from Berlin and was fascinated by them. She persuaded Hahn to investigate these results and isolate the transuranic elements. Her lab had the necessary equipment, and she duplicated Fermi’s experiments on Uranium to study the nuclear processes involved. Confirming this experimentally was no easy task. It required theoretical depth and precise rigorous experimental skills in physics and chemistry. The Study Begins
The experimental setup was simple but effective. A uranium sample, the target material, was placed under a source, which emitted a steady stream of neutrons. The expectation was, as Fermi had hypothesized, that uranium atoms would absorb these neutrons and decay into heavier elements. To track these changes, the researchers used chemical separations and radioactive decay analysis, carefully isolating reaction products based on their chemical behaviour.
It was painstaking work. The samples contained a complex mix of reaction products, including polonium, radium, thorium, and elements resembling tantalum. By tracking half-lives and decay patterns, they gradually mapped how uranium was transforming.
Meitner and Hahn were later joined by Strassmann, forming what became known as the Berlin group. Meitner focused on physics interpretations, while Hahn and Strassmann carried out intricate chemical separations.
Their experiments expanded beyond uranium, using slow-moving neutrons to bombard various elements. They assumed that when a uranium nucleus (92) absorbed a neutron, it would decay into heavier elements, first element 93, then 94, and so on.
However, the results contradicted their expectations. Strassman’s chemical analyses failed to confirm the presence of transuranic elements.
Instead, he observed that some reaction products behaved chemically like barium (56), a much lighter element. This was highly unexpected as uranium was far too heavy to break down into something as small as barium.
At first, Hahn suspected the product might be radium (88), which is chemically similar to barium (56). However, further tests confirmed that it was barium itself. While the chemical evidence was undeniable, Meitner and other physicists found the result theoretically baffling. No known process could explain how uranium had split into such a light element.
In the meantime, while Hahn and Strassmann refined their chemical analyses, there was a growing antisemitic regime in Germany due to which Meitner was advised to leave Berlin to escape persecution, much against her wishes. Her escape from Berlin to Stockholm, Sweden was dramatic and nail biting. She was accompanied by a Dutch physicist Dirk
Coster. He had diplomatic connections to help Meitner cross the German- Dutch border because travelling was risky for Jewish people under the Nazi regime. Once she reached Sweden, she set up her research and throughout this tumultuous time continued her work with Hahn and Strassmann advising them to fine tune their experiments and analyses.
She wrote letters to them and occasionally spoke on the telephone. One of the reasons that the story of nuclear fission is so well documented is the copious written communication between Meitner and Hahn. Meitner, now in Sweden, worked on the theoretical implications of their findings. What they had observed was unprecedented: the nucleus had not just absorbed a neutron but had undergone a complete rupture. A deeper explanation was needed. Hahn was confused and resistant. Chemically, barium and uranium were worlds apart in the periodic table. There was no way that uranium (238), a massive atom, could simply shatter into something as light as barium, at least, not according to the chemical principles Hahn understood.
A Light at the End of the Tunnel
By December 1938, Hahn and Strassmann obtained clear evidence that their uranium samples had broken into much lighter elements, specifically barium (56).
On December 19, 1938, Hahn wrote to Meitner that the results of the experiments were ‘peculiar’. He explained that the (supposed) radium (88) acted like barium (56), much to his surprise. As a chemist, he was sure that uranium could not actually burst into barium, which is so much smaller. Such a drastic change in the uranium nuclei puzzled Hahn. He wrote to Meitner requesting her help in understanding how these results could be possible. But without waiting for her reply, he went ahead and published his findings. When Meitner received Hahn’s letter on December 21, she responded instantly: “Your radium results are very puzzling. A reaction with slow neutrons that supposedly leads to barium!... At the moment the idea of such a largescale breakup [of the uranium nuclei] seems very difficult to me, but in nuclear physics we have experienced so many surprises that one cannot unconditionally say, ‘It is impossible’”.
Based on Meitner’s encouragement and response, on December 27, Hahn added a vital new paragraph to his paper, suggesting that the nucleus of Uranium had actually split. Even after publishing his findings, Hahn remained unsure, believing that this suggestion was a ‘fantasy’. A Christmas Gift
Meanwhile, Meitner was in Kunglav, Sweden, where she was a refugee. Without her lab, without any research at hand, she felt lost. But she continued thinking about Hahn’s puzzling results. She invited her nephew, Otto Frisch, who was also a nuclear physicist, to visit her for Christmas. Frisch was fond of his aunt and always welcomed the opportunity to meet her. He had so much to learn from her. They met in Kunglav, which was completely covered in dense winter snow. Frisch found that Meitner was completely immersed in Hahn’s questions all the time. It’s all she could think and talk about.
The notion that bombarding uranium with a neutron could produce a much smaller element seemed impossible. Hahn, Strassmann, the Joliot- Curies, and Fermi were all brilliant scientists, and their well-supported theories made it difficult to accept an idea that defied established physics. Nuclear reactions had only ever emitted tiny particles like neutrons, electrons or helium nuclei (alpha particles), never something as large as barium. For barium to appear, uranium’s nucleus had to split, but calculations showed that breaking a nucleus into large fragments required immense energy. Meitner trusted Hahn’s chemical expertise more than prevailing physical theory. She couldn’t ignore what he had found.
After breakfast, Frisch invited Meitner to ski with him, which she declined. She preferred to walk while he decided to go ahead with skiing. Walking with impressive pace along with him, she was almost 60 years old then, she discussed Hahn’s letter with him and together they tried to come up with a possible explanation of Hahn’s results.
In the quiet of the snow-covered woods, Meitner and Frisch pondered the puzzle before them. Resting for a moment, Meitner pulled out a pencil and paper. Rutherford’s model of the atom wouldn’t work, there was no way a solid uranium nucleus could split to form something as small as barium. But Bohr’s model offered a possibility, if the usual energy calculations were wrong.
She sketched a raindrop to represent the uranium nucleus, first as a sphere, then stretching into an elongated shape, wobbling like a spring before splitting into two smaller drops. If the nucleus behaved like this, the forces within it might shift in ways physicists hadn’t yet considered. She recalled the complex formula governing nuclear forces and began adjusting it to match her new assumption.
As she worked, Frisch later recalled, they realized that the uranium nucleus, with its immense charge, was already on the verge of instability. A single neutron would be enough to tip it over the edge, splitting it apart. Meitner calculated the energy released in such a split, using Einstein’s E=mc². The loss of mass, about one-fifth of a proton, translated into 200 megaelectron volts (MeV), exactly the amount needed for the two new nuclei to separate.
Everything suddenly fell into place. They sat in stunned silence, staring at the numbers. As they continued walking, Meitner paused, suddenly grasping the full implication: “Bohr was right! The atom must be like a liquid drop.” Her sketches, calculations, and reasoning explained how uranium could split into barium, perfectly matching Hahn’s experimental results. That evening, they shared a quiet Christmas dinner with friends, then resolved to publish their findings together.
This way, Lise Meitner and Otto Frisch arrived at the first theoretical explanation of nuclear fission. While Frisch was delighted at this breakthrough, but it left Meitner stunned. As surprising as it may have been, and positively so, this explanation also refuted four years of her work on transuranic elements.
Closing Thoughts
“Oh, what idiots we have all been! Oh, but this is wonderful! This is just as it must be…” is what Neils Bohr is said to have exclaimed when Frisch told him about this discovery (Frisch, 1967). Having made the initial breakthrough, Frisch and Meitner collaborated over long-distance telephone calls. They came up with an experiment to test their explanation. Eventually, in January, they published their findings and confirmed the theory of a new nuclear process: nuclear fission. Frisch named the new process ‘fission’ after learning that the term ‘binary fission’ was used by biologists to describe cell division.
Thus, two separate papers were published about the discovery of nuclear fission. One in the field of chemistry by Hahn and Strassmann, and one in theoretical physics by Meitner and Frisch. This discovery came after nearly forty years of investigation into the nature and properties of radioactivity and radioactive substances which challenged several assumptions in physics and chemistry. The conclusion that such an unusual reaction can occur signalled the end of a rollercoaster episode in the history of science and led to further investigations. Almost immediately, dozens of labs across the world confirmed these findings. More than 100 papers were published within a year of the discovery, describing important features of the process and confirming the formation of heavy energy particles. Here is an infographic that summarizes this interesting and complicated journey of discovery.
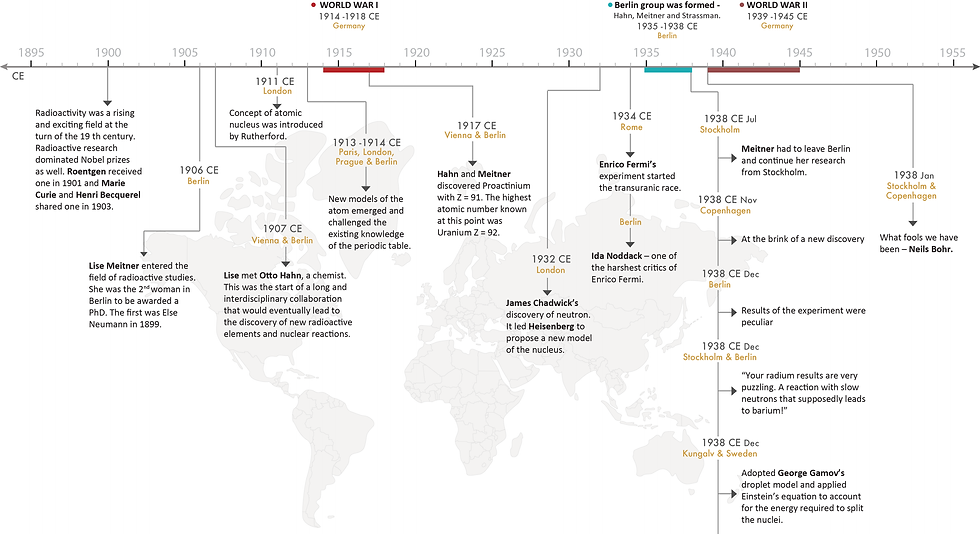
This story would be incomplete without mentioning that Meitner was never made the co-author of the findings of nuclear fission in the paper published by Hahn and Strassmann. There is a great deal of documentation to suggest that Hahn distanced himself from here during World War II owing to her Jewish origins. Hahn received the Nobel Prize in Chemistry for the discovery of nuclear fission. Meitner, though she was nominated, for a Nobel Prize in Physics that year, did not win. And this rejection would be just one, among the 47 times that she was rejected for a Nobel prize. Yes, she was nominated 47 times – 19 times for Chemistry between 1934 and 1948 and 29 times for Physics – between 1937 and 1965.
Meitner never ever allowed her rejection and setbacks, be it for her race of gender, to lose focus on her science. She cared about it deeply and continued to contribute and publish till her dying day.
The title A Large-Scale Breakup is a metaphor for both scientific and personal separation. Just as the discovery of nuclear fission shattered the idea of an indivisible nucleus, it also marked the split of a four-decadelong collaboration between Meitner and Hahn. Once bound together like protons and neutrons in an atomic nucleus, their partnership was fractured by political upheaval, scientific competition, and the very discovery they had worked toward.
Lise Meitner’s pioneering work in nuclear physics is yet another story of how women in STEM face systemic discrimination. In 1964, sociologist Robert K. Merton introduced the term Matthew Effect to describe how well-known scientists often receive more credit than lesser-known peers, even when the work is collaborative. This phenomenon is more commonly known today as the Matilda Effect, a term coined by science historian Margaret Rossiter in 1993, named after Matilda Joslyn Gage, a suffragist and abolitionist who advocated for women's rights.
*End of Story*



Comments